Mepilex XT in practice
Various wounds, including venous leg ulcers, responded well to Mepilex® XT – and the dressing effectively managed different types of wound exudate in an eight-month study.
Mepilex XT in practice: results of a study in German specialist wound care centres
Lantin A, Diegel C, Scheske J, Schmitt C, Brönner A, Jodl H. Wounds International 2015; 6(4):18-22
- Effective exudate management has been shown to contribute to a reduction in time to wound healing, a reduction in skin damage and risk of infection, whilst improving patient quality of life and healthcare efficiency.
- It is important that clinicians find a balance between the beneficial and damaging effects of wound exudate; this will help to achieve moist wound healing, while minimising the risk of moisture-related damage to the wound and periwound skin.
- Focus should be on the nature of wound exudate as well as exudate volume; the clinical and economic challenges posed by different exudate types should be considered when developing an exudate management plan.
- Mepilex XT incorporates exudate channels, designed to draw both low and high viscosity exudate away from the wound bed, helping to protect the wound and peri-wound area from moisture-related damage.
Aims
To evaluate the performance of Mepilex XT in clinical practice, when used as part of the wound management regimen in a variety of wound types.
Methods
- Registry study, conducted across 10 specialised wound care centres in Germany; all of the centres work with the same standard treatment path and use a digital wound documentation system.
- Mepilex XT was used across all 10 wound care centres over an 8-month period.
- A number of different wound types were included in the study, including arterial/mixed/venous ulcers, traumatic wounds, diabetic/neuropathic ulcers, decubitus ulcers and neoplastic wounds. These wounds were located in a variety of anatomical locations.
- For each wound treated with Mepilex XT, data captured at each scheduled dressing change (over a period of up to 6 weeks) were analysed; typically, patients are seen at the clinic every 2 weeks.
- A number of wound-related and dressing-related parameters were tracked (Table 1).
Table 1: Scope of data extraction from digital wound documentation system for inclusion in the analysis
| Wound-related parameters |
| Types |
| International Classification of Diseases (ICD-10) |
| Age at start of treatment |
| Location |
| Size |
| Condition |
| Exudate |
| Signs of infection |
| Dressing-related parameters |
| Treatment duration |
| Wear time/interval between dressing changes |
| Ease of dressing changes |
| Pain at dressing changes |
- During the evaluation period, 136 patients (52.2% female; 47.8% male) had wounds appropriate for treatment with Mepilex XT; wounds with no exudate were not considered suitable for treatment with the dressing.
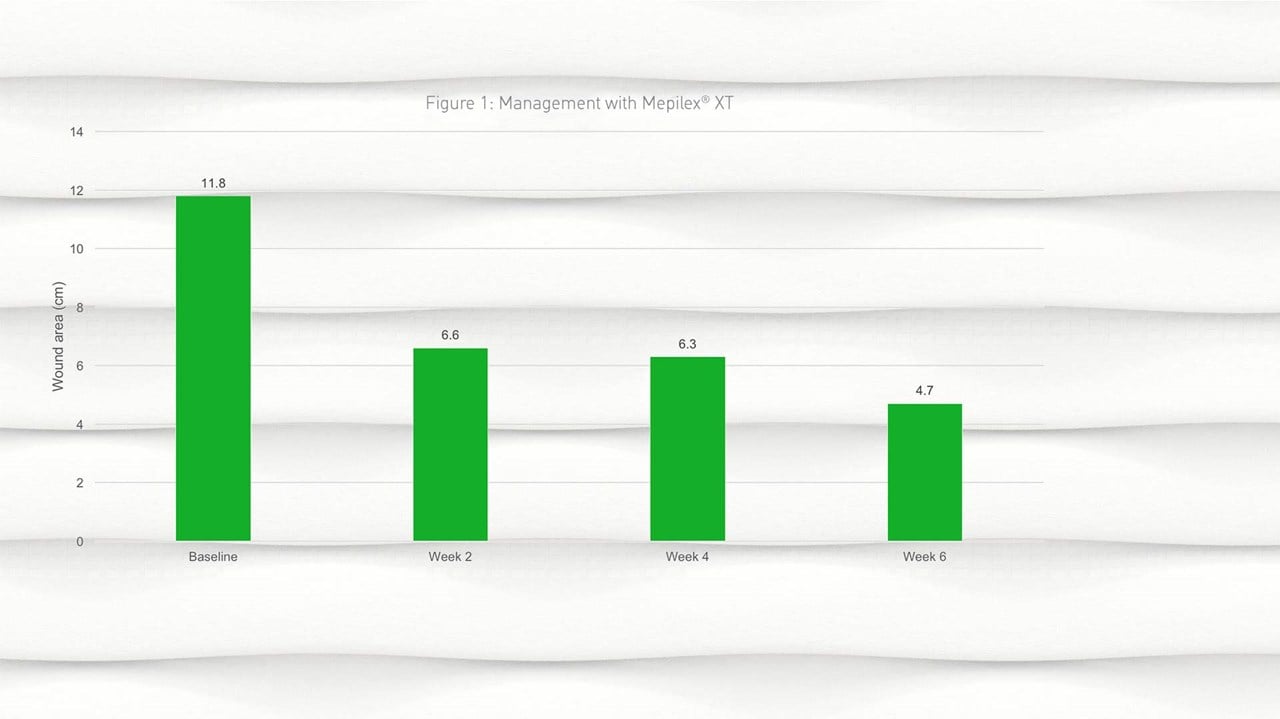
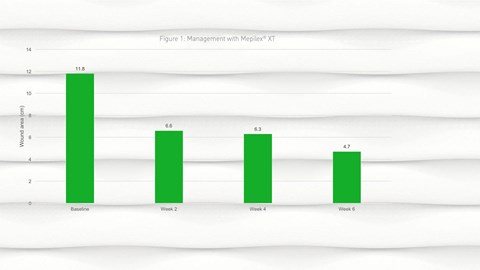
- By week 2, a reduction in wound size was observed from a mean of 11.8cm2 (SD 20.62) at baseline to 6.6cm2 (SD 10.96) at week 2; this reduction in wound size continued into week 4 and week 6 (Figure 1).
- From baseline to week 6 of treatment, a healing rate of 31% was observed.
- Mepilex XT dressings were observed to effectively handle wound exudate, irrespective of the quantity, type and/or viscosity.
- No observation of leakage or maceration was reported throughout the study.
- Median total treatment duration with the dressing was 39 days (range 7-309 days); during this treatment duration, the median number of dressing changes per week was 3 (range 1-7).
- At dressing changes, Mepilex XT dressings were found to be easy to remove in over 85% of dressing changes.
- An overall reduction in pain severity at dressing change from baseline to week 6 was observed (pain data based on 18 patients, as other patients did not experience pain at all at dressing change).
- During this study, those wounds treated with Mepilex XT generally demonstrated a good healing response in terms of a reduction in wound size.
- The dressing effectively managed different types of wound exudate, including high viscosity exudate. As such, the dressing demonstrated its potential to be used throughout the various wound healing stages.
- The dressing also demonstrated relatively long wear times, indicating potentially positive implications on cost-effectiveness.






