Patient and Wound History
- A 73-year old male presented with a diabetic neuropathic foot ulcer (DFU)
- The patient had a current medical history of type II diabetes, chronic obstructive pulmonary disease (COPD) and osteomyelitis treated with antibiotics (clindamycin and ciprofloxacin). There was no surgical
history - The ulcer, located on the apex of the right first toe, measured 1089mm2 with a depth of 2mm to the bone. The ulcer had been present for a duration of 5 months
- The wound bed was composed of 80% granulation tissue, 19% slough and 1% exposed bone
- Clinical signs of infection included increased wound warmth and an increase in wound exudation
- Exudate levels were moderate and were serosanguinous/blood in nature
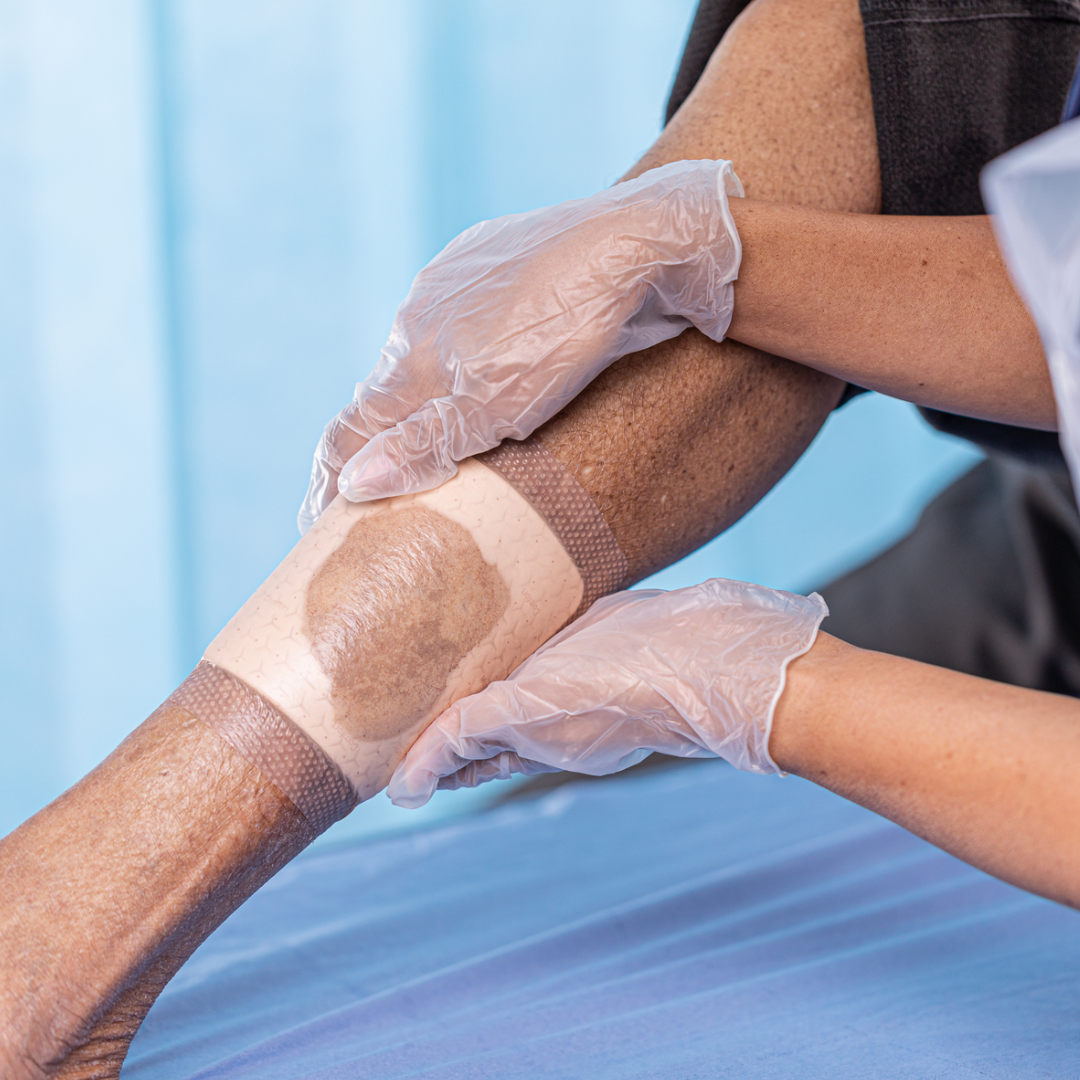
- The peri-wound skin was healthy and intact
- The wound had previously been treated with UrgoTul® Absorb Border; a surgical shoe with total contact insole provided off-loading

Intervention and Treatment Regime
- Treatment with Mepilex® Border Comfort was monitored over a 25-day period, during which the patient attended 4 follow-up visits at the podiatry clinic
- At the follow-up visits, the dressing was changed. Dressing changes were performed according to local clinical practice or when the dressing became saturated. Dressings were always changed at each of the clinic visits; in between the scheduled visits, dressings were changed at the discretion of the patient / clinician
- A total of 11 Mepilex® Border Comfort dressings were used during the study period; the median dressing change frequency was 2 days (range 1 – 4 days)
Follow-up assessments
- Over the period of investigation, the wound steadily decreased in size
- At the initial follow up visit, the condition of the wound bed had improved slightly (85% granulation tissue, 14% slough and 1% bone), but thereafter remained unchanged
- At all follow-up visits, clinical signs of local wound infection were absent
- The peri-wound skin remained healthy and intact throughout the study period
- Exudation was recorded as moderate and serosanguinous/blood in its nature; at the final two visits a slight decrease in exudate level was noted
- The patient did not experience pain during the dressing change procedures
- Over the treatment period, the condition of the wound improved and at the final follow-up visit the wound bed was composed of 98% epithelial tissue and 2% granulation tissue
Wound Progression

Clinical outcome
- After 25 days of treatment with Mepilex® Border Comfort, the wound measured 625mm2, a 43% reduction by area, but wound depth remained unchanged. The condition of the wound bed was stable, and the peri-wound skin was healthy
- The podiatrists rated Mepilex® Border Comfort, on average, as 'Excellent’ in terms of its handling ability, ease of application, stay-on, ability after application, ability to allow multiple inspections of the wound without loss of adherence, exudate handling capability and ease of removal without pain or skin damage
- The podiatrists observed that Mepilex® Border Comfort conformed well to the wound in an area difficult to dress; Mepilex® Border Comfort ‘stays in position better than previous dressings that were being used’
- Upon commencing treatment with Mepilex® Border Comfort, the patient stated that the wound pain he had experienced previously between dressing changes, especially at night time, was absent. The patient reported the dressing change procedure to be very easy
Acknowledgement: Photographs and case notes kindly supplied
by Samantha Haycocks, Advanced Podiatrist, Salford Royal NHS Foundation Trust, Salford, United Kingdom
Learn more about Mepilex® Border Comfort
Our next generation of flexible dressings are designed to stay on and uniquely conform; giving time back to nurses, cost savings back to managers and quality of life back to patients.




