Patient and wound history
- An 81-year-old female presented with a traumatic skin tear following a fall in her home.
- The patient who was registered blind, had a current medical history of type II diabetes mellitus, hypertension, heart disease, vitamin D deficiency (all prescribed medication) and osteoarthritis.
- There was no surgical history.
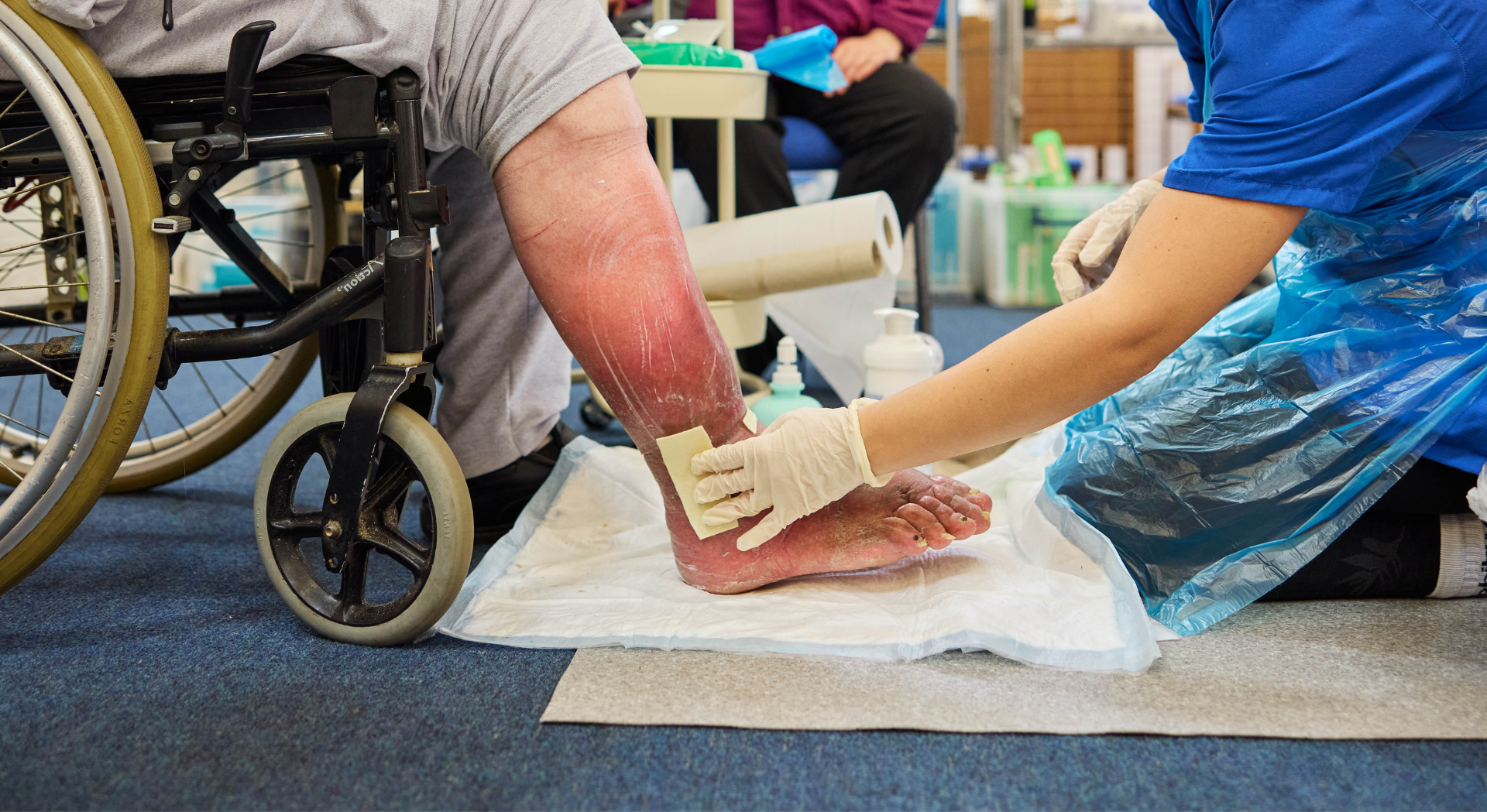
- The wound, located on the right gaiter region, measured 71.5cm2 with a depth of 0.5cm, and had been present for 1 week.
- The wound bed was composed of 10% granulating, 80% sloughy and 10% necrotic tissue.
- The wound presented with mild, increased warmth and pain, and a moderate increase in exudation, all indicative of wound infection. No antibiotics were prescribed.
- Exudate levels were high; viscous and yellow/green in appearance.
- The peri-wound skin was extremely fragile and dry.
- Initially, the wound was treated with steri-strips and a secondary dressing. On referral to the district nursing team, the loose steri-strips were removed and Silvercel (silver-containing alginate dressing) applied.
- At baseline, pain resulting from the action of touching the wound during the dressing change procedure was recorded as 5, as measured on a visual analogue scale (VAS) ranging from 0 (no pain) to 5 (maximum pain ever). Upon application of Exufiber® Ag+, a VAS score of 5 was recorded

Intervention and treatment regime
- Autolytic debridement of the wound via Exufiber® Ag+ was observed during the initial 20 days of treatment. In addition, the wound was cleansed and debrided using UCS wipes throughout the study.
- During the first 20 days of the study, the wound was dressed with Exufiber® Ag+ (primary dressing) and Zetuvit Plus (superabsorbent dressing; secondary dressing).
- After 4 weeks of treatment, wound infection had resolved, and the primary
dressing was switched to Exufiber®. During the final 4 weeks of the study, PolyMem® (polymeric membrane dressing) was applied to the wound. Throughout the study period, KTwo® Reduced Compression bandages were applied. - The patient attended 3 follow-up clinic visits
- At each follow-up visit, the dressings were changed; in between the scheduled visits, dressings were changed twice weekly over the initial three weeks, and thereafter dressings were changed weekly. All dressing changes were performed according to local clinical practice.
- The wound dressings were changed 14 times during the study period; the median dressing change frequency was 7 days (range 3 - 7 days).
Follow-up assessments
- Over the study period, the wound size reduced significantly, and at the final follow-up assessment the wound had healed.
- The composition of the wound bed tissue improved substantially after the initial 20 days of treatment with Exufiber® Ag; 80% granulating and 10% epithelialising tissue were present, and just 10% sloughy tissue remained. At the final assessment, the wound bed was composed of 5% granulating and 95% epithelialising tissue.
- After 49 days of treatment, all clinical signs of wound infection had been resolved.
- Wound exudate levels steadily reduced during the treatment period, and at the final follow-up visit, exudation was absent.
- At the final study assessment, the peri-wound skin remained dry and extremely fragile.
- After 20 days of treatment with Exufiber® Ag, pain when the wound was touched (all points during dressing change) was reduced to a VAS score of 3. Thereafter the patient experienced no pain. Following application of the new dressings, pain was reduced at the initial two follow-up assessments, with VAS scores of 3 recorded, thereafter the patient was pain-free.
Wound progression

Clinical outcomes
At the final evaluation, the wound had healed.
The clinical team (TVN and district nurses) were very impressed with how Exufiber® / Exufiber® Ag+ handled the wound exudate, especially its viscosity.
The TVN was ‘gobsmacked’ that the wound progressed to heal as the patient had such a complex past medical history with many additional health issues.
Early in the treatment period, the patient conveyed that she noticed a reduction in ‘niggling’ wound pain.
Acknowledgement: This case study report has been prepared by Mölnlycke’s Global Medical Affairs & Safety team, based on information and photographs kindly supplied Debbie Johnstone, Tissue Viability Nurse, Bradford District Care Foundation Trust, Bradford, UK who has also confirmed and given Mölnlycke permission to distribute this report
Learn more about Exufiber® & Exufiber® Ag+
Exufiber® is designed to manage exudate efficiently and stay intact for clean, one-piece removal.




