Patient and Wound History
- A 77-year-old female presented for same-day management with an acute traumatic skin tear (Type 2).
- The patient has a current medical history of asthma
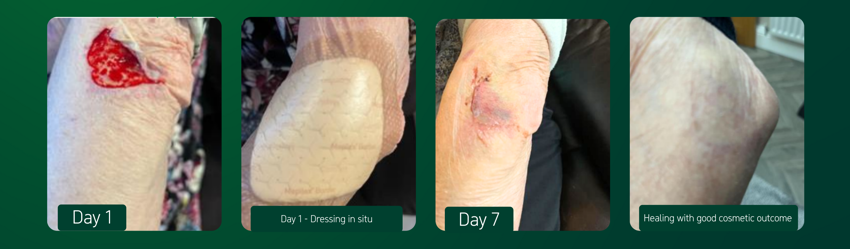
- The wound, located on the elbow of the right arm, measured 7cm x 7cm.
- The wound bed was composed of 100% denuded dermis.
- The wound exudate level was low; serosanguinous in appearance.
- The peri-wound skin was fragile.
- At baseline, pain prior to receiving treatment was recorded as 8, as measured on a visual analogue scale (VAS) ranging from 0 (no pain) to 10 (maximum pain ever). Upon dressing application, a VAS of 8 was recorded.
Treatment Regime
- A detailed wound evaluation was completed at a baseline assessment and at a scheduled study visit on treatment day 7.
- Prior to dressing application, the skin flap was gently eased, as close as possible, back over the wound without applying tension. The wound area was then dressed with Mepilex® Border Comfort dressing.
- The wound dressing was changed at day 3 according to local clinical practice.
- At the dressing changes, the wound was cleansed with saline.
Follow-up assessments
- After 7 days of treatment, the dressing had stabilised the replaced skin flap. The edges were completely re epithelialised and bruising had reduced to the centre of the flap.
- At the final study evaluation, wound exudate was absent.
- The skin covering the healed wound and the peri-wound skin remained
fragile. - At the final evaluation, the patient had no pain prior to or during dressing removal.
Wound Progression

Clinical outcome
- The primary approach to managing this patient was to facilitate faster healing.
- At the final evaluation, the wound had healed but the new epithelial tissue was fragile.
- The clinician was impressed that the objectives for the patient were met in
such a short period of time. The patient was able to shower daily keeping the dressing in situ. When removed, Mepilex® Border Comfort was atraumatic. - The patient reported Mepilex® Border Comfort was comfortable to wear with no restriction of arm movementAfter 25 days of treatment with Mepilex® Border Comfort, the wound measured 625mm2, a 43% reduction by area, but wound depth remained unchanged. The condition of the wound bed was stable, and the peri-wound skin was healthy
Acknowledgement: This case study report has been prepared by Mölnlycke’s Global Medical Affairs & Safety team, based on information and photographs kindly supplied by the patient’s clinician who wishes to remain anonymous but who has confirmed and given Mölnlycke permission to distribute this report,
Learn more about Mepilex® Border Comfort
Our next generation of flexible dressings are designed to stay on and uniquely conform; giving time back to nurses, cost savings back to managers and quality of life back to patients.





